Ozone therapy has possible mechanisms for treating ALS. A preclinical study in very small numbers of mTDP43 mice (which has yet to be peerreviewed) suggested benefits on motor function and survival (21,22); however, these benefits were not seen in mSOD1 mice (20). One verified “ALS reversal” occurred on a cocktail of alternative therapies including ozone (24); an association such as this does not prove causality. There have been no trials of ozone therapy in PALS. There may be potentially serious side effects associated with ozone therapy, depending on the dose (31). Based on all this, we support further investigation of ozone therapy in ALS cell or animal models, but we cannot yet recommend it as an ALS treatment.
Pre-clinical models (animal or cell models recognized by ALSUntangled reviewers to be relevant to ALS)
Rituximab
ALSUntangled reviews alternative and off-label treatments on behalf of people with ALS who ask about them. Here we review rituximab, a drug which specifically depletes B lymphocytes. We show a current lack of evidence for a role of these cells in ALS progression. The one patient we found who described using Rituximab for their ALS found no benefit. Given all this, and the known serious risks of rituximab, we advise against its use as an ALS treatment.
You can read it here
https://www.tandfonline.com/doi/full/10.1080/21678421.2022.2122845?src=
Acetyl-L-Carnitine
There are good theoretical mechanisms for carnitines, some pre-clinical evidence for LC and ALCAR, and a single clinical trial that suggested ALCAR could slow disease progression in PALS. All three carnitines appear to be well-tolerated, generally safe, and inexpensive. We believe that there is a need for future clinical trials of carnitines in PALS to further elucidate their efficacy. Until there is further data, we cannot endorse any of these supplements as a definite way to slow ALS progression; however, oral ALCAR at 1000mg three times daily (3000 mg total daily dose) appears to be a theoretically promising supplement available for PALS whom would like to self-experiment.
GM604
At this time ALSUntangled finds no independently verifiable data supporting the efficacy or even the safety of GM604 in patients with ALS. We believe that independent peer review and replication are fundamentals of good science (36,37). Accordingly, we share the FDA’s April 2015 opinion that the data on GM604 in ALS should be released now for
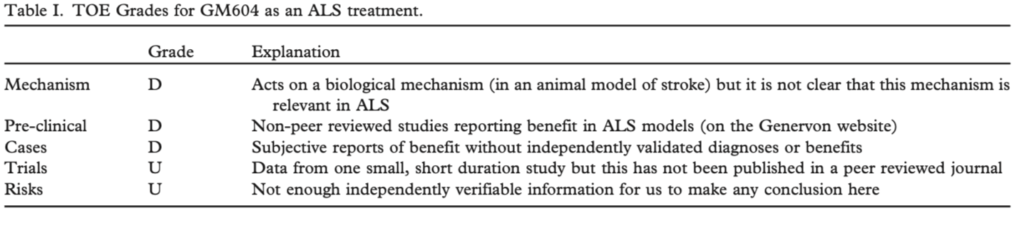
independent peer review (38). If these preliminary data are confirmed to be positive, statistics on the false-positive rate of small trials (29,30) and consensus ALS trial guidelines (35) dictate that they be replicated in a larger, longer duration study before GM604 is deemed effective or even safe for patients with ALS.
ALSUntangled generally supports the use of expanded access programs during ALS drug development. We believe that these should be reserved for treatments that have at least some independently verifiable safety data. In our opinion, that is not the case with GM604, so we feel that expanded access is premature at this time. When we can independently verify safety data, we hope to see a GM604 group expanded access program that has transparent entry criteria, systematic objective outcome measures, full disclosure of results, and, as suggested by the FDA, allows for a sponsor’s cost recovery but not for profit (39).
Click here to download the complete review.
Sodium Chlorite WF10
The NP001 formulation of sodium chlorite acts through a plausible mechanism and preliminary data suggest that it is safe and may slow ALS progression in some PALS. The WF10 formulation of SC appears to act through this same mechanism. Although WF10 is available for off-label use, it is very expensive, may have more side-effects than NP001, and at this time has only scant anecdotal evidence for efficacy in PALS. ALSUntangled supports further carefully monitored studies of NP001 and WF10 in PALS. In contrast, oral sodium chlorite has potentially dangerous and toxic side-effects may hasten disease progression, and is not clearly absorbed from the gut. We do not recommend further use of oral sodium chlorite unless it can at least be shown to be safe and to act on mechanisms in humans that are relevant to ALS.
Sodium Chlorite NP001
The NP001 formulation of sodium chlorite acts through a plausible mechanism and preliminary data suggest that it is safe and may slow ALS progression in some PALS. The WF10 formulation of SC appears to act through this same mechanism. Although WF10 is available for off-label use, it is very expensive, may have more side-effects than NP001, and at this time has only scant anecdotal evidence for efficacy in PALS. ALSUntangled supports further carefully monitored studies of NP001 and WF10 in PALS. In contrast, oral sodium chlorite has potentially dangerous and toxic side-effects may hasten disease progression, and is not clearly absorbed from the gut. We do not recommend further use of oral sodium chlorite unless it can at least be shown to be safe and to act on mechanisms in humans that are relevant to ALS.
Hyperimmune Goat Serum for ALS
The mechanism of Aimspro remains unproven; if it is an immunomodulator and/or a modulator of sodium channels, it theoretically could be useful in ALS. A single, detailed but significantly flawed case report documents slowing in decline of certain respiratory functions in a patient claiming to have ALS, who started Aimspro shortly after bipap. Based upon this limited information, ALSUntangled supports further study of Aimspro, either in ALS animal models or in a small phase 2 trial with clear and objective endpoints carried out by skilled trialists familiar with the problems inherent with ALS clinical studies. Until a trial is undertaken, however, we do not support further use of this product by PALS.