Since our last review we found a new paper suggesting that GM604 can modify the expression of a large number of genes in neuroblastoma cells
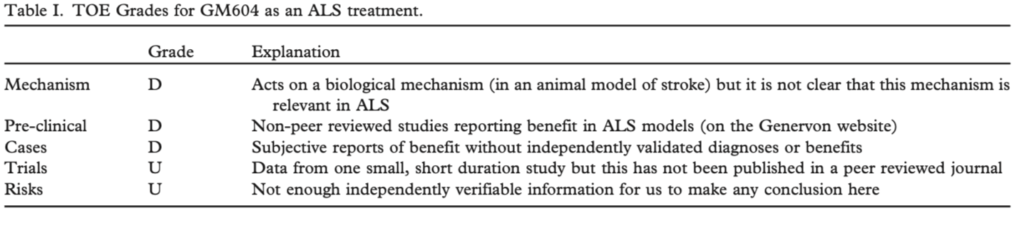
(Swindell et al. Translational Neurodegeneration (2018) 7:30). These cells are not a recognized pre-clinical model for ALS. The described genes have not been proven to influence human ALS progression. Our “Mechanisms” Grade therefore remains unchanged. We also found an Internet publication describing a case report and a very small (12 patient) clinical trial (F1000Research 2017, 6:230 Last updated: 17 JUL 2018). The case report suggested benefits from GM604 treatment in 1 person with ALS, but no validated objective ALS outcome measures were described. We therefore cannot change our current “Cases” grade. The trial compared 2 weeks of treatment with GM604 to placebo, with a total of 12 weeks of follow up. No statistically significant differences in ALSFRS-R, FVC, TGUG, grip strength or HHD scores were observed between these groups at week 12. The authors of this paper, (one of whom is an executive at the company that sponsored the trial) state that the GM604 treated group progressed more slowly than a historical control group, but it is not clear to us that the historical controls were adequately matched with regard to baseline prognostic factors. A post-hoc subgroup analysis was said to show benefits of GM604 treatment on FVC for the 4 patients at one of the 2 sites. It is not clear to us that this is a scientifically valid subgroup. Multiple “biomarkers” changed in response to GM604 treatment, but it is not clear to us that any of these are a useful way to measure ALS progression. Based on this one publication, we change our “Trials” grade to F. We note that this trial was underpowered and probably too short to detect modest changes in the clinical outcomes selected. Safety and tolerability were said to be good in the participants in this trial but we feel this is too small a number and too short an exposure to change our “Risks” grade. Our overall conclusion remains unchanged. We hope to see more data on GM604 in patients with ALS. Until this arrives and confirms benefits and safety, we cannot endorse this as an ALS treatment.
Key Information
At this time ALSUntangled finds no independently verifiable data supporting the efficacy or even the safety of GM604 in patients with ALS. We believe that independent peer review and replication are fundamentals of good science (36,37). Accordingly, we share the FDA’s April 2015 opinion that the data on GM604 in ALS should be released now for
independent peer review (38). If these preliminary data are confirmed to be positive, statistics on the false-positive rate of small trials (29,30) and consensus ALS trial guidelines (35) dictate that they be replicated in a larger, longer duration study before GM604 is deemed effective or even safe for patients with ALS.
ALSUntangled generally supports the use of expanded access programs during ALS drug development. We believe that these should be reserved for treatments that have at least some independently verifiable safety data. In our opinion, that is not the case with GM604, so we feel that expanded access is premature at this time. When we can independently verify safety data, we hope to see a GM604 group expanded access program that has transparent entry criteria, systematic objective outcome measures, full disclosure of results, and, as suggested by the FDA, allows for a sponsor’s cost recovery but not for profit (39).
Click here to download the complete review.
