Key Information
Now 26 months old, ALSUntangled (www.alsuntangled. org) investigates alternative and off-label treatment options in ALS using social networking tools. Our twitter and NING memberships continue to grow. To date we have published 10 investigations on 11 different alternative and off-label treatment options, collaborating with Quackwatch (www. quackwatch.org), Patients Like Me (www.patients likeme.com) and ALS Worldwide (www.alsworld wide.org). We now present our investigation of NuTech Mediworld’s ALS treatment regimen which was undertaken at the request of patients with ALS (PALS).
NuTech Mediworld is a ‘stem cell clinic’ in New Delhi, India and is run by Dr. Geeta Shroff. In an effort to obtain information about the clinic, we tried to start with the clinic website (www.nutech mediworld.net), but this site has been down for the entire three months that we have been working on this piece. We made three attempts at contacting Dr. Shroff via email at the address we found on the web (1); unfortunately, we received no responses. We performed a search of the literature using Pub Med; we could not find any articles published by Dr. Shroff. She has apparently applied for patents related to the use of human embryonic stem cells used in a variety of seemingly vague ways to treat more than 70 human diseases including motor neuron disease (2). Blogs, YouTube videos and various interviews with patients with ALS suggest that Dr. Shroff is an ‘infertility expert’, using a cell line derived from a single embryo, and that these are being injected multiple times into the spinal fluid (2). In an interview Dr. Shroff suggested that, as of 2007, she had treated “more than 300” patients and that “almost everybody” had improved (3). In that same interview, it was noted that she was not using “any immunosuppressants or anti-rejection drugs” along with her treatments (3). Beyond this, we have been unable to find any presentation of an actual ALS treatment protocol, or any detailed safety or efficacy data.
We found two PALS who received treatment at Nu tech Mediworld and were also cared for by ALSUntangled affiliated clinicians. The first was a 44-year-old male, diagnosed in early 2010. He started regular follow-ups with an ALS expert only after his trip to NuTech Mediworld in June 2010, so it is not possible to examine validated outcome measures before and after his treatment there. How- ever, he provided a discharge summary from NuTech Mediworld with Dr. Geeta Shroff listed as ‘doctor in charge’. Interestingly, this contains no record of the patient’s prior work-up; furthermore, the only neurological examination states that the patient was ‘conscious, cooperative alert, oriented with time, place’. Thus, it does not appear that any objective confirmation of his ALS diagnosis was made. No consent form was provided with the discharge summary. Some testing was reportedly performed in the clinic. This included a blood count, serum chemistry, ‘Australia Antigen’, HIV, urinalysis, B12, vitamin D level, chest X-ray, EKG, abdominal imaging, echocardiogram, and brain SPECT scan. Under ‘treatment given’ the document merely states ‘patient was given human embryonic stem cell therapy during his stay in this hospital’. His ‘condition during discharge’ was noted to be ‘satisfactory’ with decreased ‘fatigue’, ‘walking, balance better’, increased ‘UE power’. Again, there are no validated measurements documented to confirm these conclusions. The patient reported receiving injections into his back, neck, shoulders and hands. Additionally, he reported receiving ‘three different antibiotics’ for ‘Lyme disease’ under Dr. Shroff. ‘Physiotherapy as advised’ was prescribed.
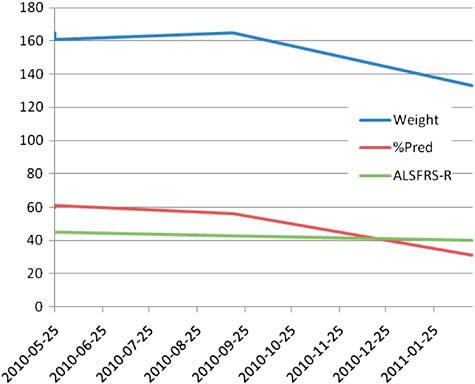
The second patient was a 36-year-old female, diagnosed in May 2010 and followed by an ALS expert before and after the NuTech Mediworld treatment. A discharge summary was not available on her, but she recalled events similar to the previous patient. These included a lack of neurological examinations, unusual testing including a Lyme test, and unusual adjunctive therapy including antibiotics for Lyme disease (even though her Lyme test was negative). Clinical data appear in Figure 1. Her weight, vital capacity and ALSFRS-R scores all appeared to worsen slowly over the first few months of her disease. Following treatment at NuTech Mediworld in late September 2010, her weight and vital capacity appeared to drop precipitously. Between May and mid-September 2010, her weight was stable and predicted vital capacity changed by only 4%. Between September and mid- February 2011, her weight dropped by 32 pounds, and her predicted vital capacity dropped by 25%. Subjectively this patient felt that her bladder control was improved by her NuTech Mediworld treatment. Her treatment cost $45,000 and was not covered by insurance (4). She did not recall any side-effects.
According to the ALS Worldwide publication ‘A Patient’s Path through the Maze of Stem Cell Transplantation’, Dr. Shroff ‘has no background in stem cells, has never published anything and makes extraordinary claims to have cured more than 100 patients of everything from Alzheimer’s disease to spinal cord injury. In fact, she claims to be able to cure more than twenty different diseases with treatments proven to be bogus. Her credentials are nowhere to be found. Multiple lawsuits against her exist’. This publication classifies NuTech Mediworld under ‘notorious stem cell operations: avoid, shun and ignore their claims’. Indeed, NuTech Mediworld and Dr. Shroff were apparently subject to a governmental inquiry in 2006; the results of that inquiry are not widely known (5).
In summary, ALSUntangled can find little objective information on the technical and safety aspects of NuTech Mediworlds’ stem cell treatment.
The main part of the treatment is alleged to be some type of embryonic cell line being injected into spinal fluid (and possibly other various body parts) without immunosuppressives; to our knowledge it has never been demonstrated that embryonic cell lines are safe, not to mention effective, for ALS when used in this way. We can find no information confirming Dr. Schroffs’ education and training to perform stem cell transplants. We can find no evidence that ALS diagnoses are being appropriately confirmed at NuTech, nor that adequate informed consent is being documented prior to treatment, nor that validated ALS outcomes are measured after treatment. We did find a number of unusual tests being performed there, as well as odd concurrent treatment for seronegative Lyme disease. For the one ALS patient in whom validated outcomes were available, there actually may have been a worsening rate of disease progression following NuTech treatment. We would welcome an opportunity to discuss our findings with Dr. Shroff and review any additional information that she can provide in order to provide as accurate and fair an assessment about NuTech Mediworld Stem cell treatments possible. We invite Dr. Shroff to write a letter to this journal, present her results at an international ALS meeting, or meet with us directly. Unless or until this occurs, ALSUntangled suggests that patients with ALS avoid NuTech Mediworld.