Considering the fact that electrical stimulation was first used as a medical treatment more than 100 years ago (30), and first used as an ALS treatment 30 years ago (31), it is disappointing that we have yet to find a clear way to use this to help PALS. PoNS™ is a newer version of this. There is a vague theoretical mechanism (neuromodulation) by which PoNS™ could potentially modulate neuroplasticity in the brainstem and cortex, but whether it provides any beneficial or deleterious effects on ALS progression is currently unknown. While there are early, promising data showing that the PoNS™ device improving gait in patients with multiple sclerosis, this may not translate to PALS. There are no pre-clinical data or clinical trials of PoNS™ therapy in PALS to determine efficacy. The PoNS™ device appears to be relatively safe but its substantial cost and prescription-only status will limit accessibility for PALS. Given the current lack of ALS-relevant data, we cannot currently support the use of PoNS™ therapy to slow, stop, or reverse ALS progression. We hope that this review of PoNS™ and the broader topic of neurostimulation spurs future research toward helping PALS.
Patient case reports
Insulin
Insulin treatment for ALS is an intriguing area for future research. However, the risks of insulin administration are significant and potentially lethal. Currently, there is no clinical evidence to support its use in PALS. Therefore, we cannot endorse insulin as a way to slow, stop, or reverse ALS progression at this time.
Proprionyl-L-Carnitine
There are good theoretical mechanisms for carnitines, some pre-clinical evidence for LC and ALCAR, and a single clinical trial that suggested ALCAR could slow disease progression in PALS. All three carnitines appear to be well-tolerated, generally safe and inexpensive. We believe that there is a need for future clinical trials of carnitines in PALS to further elucidate their efficacy. Until there is further data, we cannot endorse any of these supplements as a definite way to slow ALS progression; however, oral ALCAR at 1000mg three times daily (3000 mg total daily dose) appears to be a theoretically promising supplement available for PALS whom would like to self-experiment.
Antiretrovirals
Antiretrovirals are a group of diverse drugs developed for HIV infections that vary widely in theoretical efficacy against HERVs, side effect profiles, and cost. HERV expression is apparently increased in some PALS; however, it is unknown if this is a
beneficial, neutral, or pathological process. Furthermore, it is not clear if ARV-targeted mechanisms such as cell infection and viral replication are taking place in PALS. Based on the lack of evidence for use of ARVs in PALS who test negative for HIV and HTLV, we cannot recommend them as a treatment for ALS. We look forward to the results of the two ongoing trials of ARVs in PALS.
Click here to download the complete review.
Basis
Basis has mechanisms of action that could theoretically be useful in treating ALS. It appeared reasonably safe in a small, short duration study of healthy volunteers and it is fairly inexpensive. However, we found no data in preclinical ALS models, no case reports, and no trials in PALS. Based on this lack of data, ALSUntangled cannot currently recommend use of Basis to slow, stop, or reverse the progression of ALS.
Click here to download the complete review.
Sodium Chlorite NP001
The NP001 formulation of sodium chlorite acts through a plausible mechanism and preliminary data suggest that it is safe and may slow ALS progression in some PALS. The WF10 formulation of SC appears to act through this same mechanism. Although WF10 is available for off-label use, it is very expensive, may have more side-effects than NP001, and at this time has only scant anecdotal evidence for efficacy in PALS. ALSUntangled supports further carefully monitored studies of NP001 and WF10 in PALS. In contrast, oral sodium chlorite has potentially dangerous and toxic side-effects may hasten disease progression, and is not clearly absorbed from the gut. We do not recommend further use of oral sodium chlorite unless it can at least be shown to be safe and to act on mechanisms in humans that are relevant to ALS.
Mototab
Given the lack of demonstrated effectiveness, and the above-documented concerns about product safety and supplier identity and reliability, ALSUntangled does not support the use of mototab
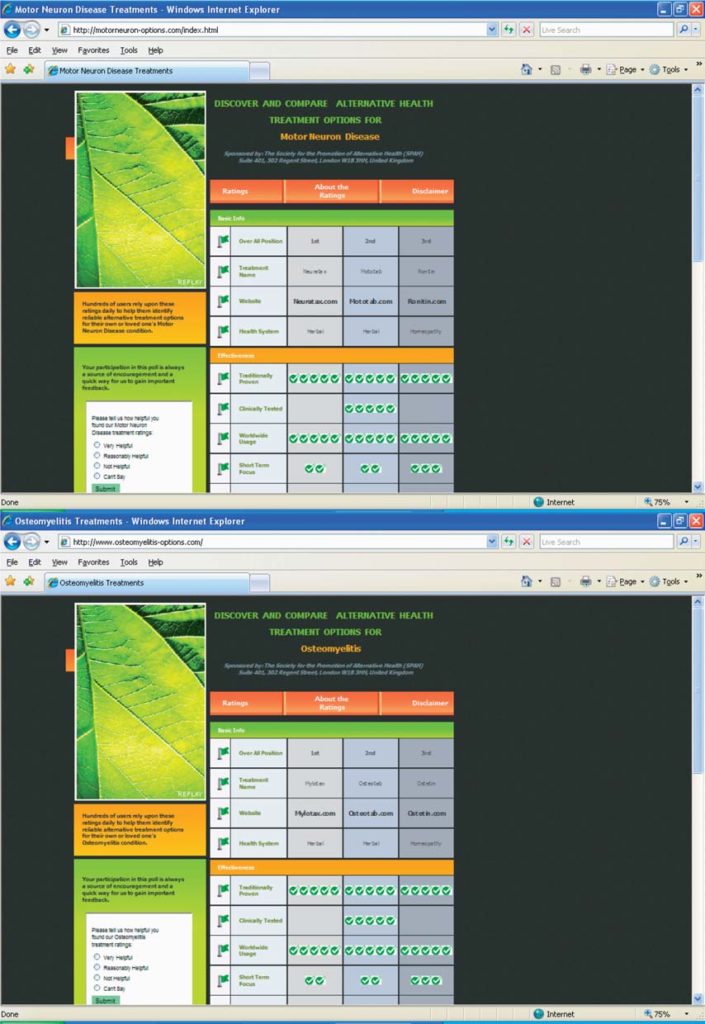
for amyotrophic lateral sclerosis or any other motor neuron disease. If Oslo Health Solutions ever con-tacts us with additional useful information on this product we will gladly publish an addendum to this investigation.
Spirulina (blue green algae) as a treatment for ALS
At this time, ALSuntangled finds no evidence that Spirulina is effective for ALS and there appear to be real and theoretical toxicities that patients with ALS may encounter with it. Until better efficacy and safety studies are published, we do not support the use of Spirulina in patients with ALS.